- Study protocol
- Open access
- Published:
An overview of implementing an evidence based program to increase HPV vaccination in HIV community clinics
BMC Public Health volume 22, Article number: 1696 (2022)
Abstract
Background
HPV-related anal cancer occurs in excess rates among people living with HIV (PLWH) and has been increasing in incidence. The HPV vaccine is an effective and safe approach to prevent and reduce the risk of HPV-related disease. Yet, HPV vaccine programs tailored and implemented in the HIV population are lagging for this high-risk group.
Methods
A pre-post intervention study design will be used to tailor, refine, and implement the 4 Pillars™ Practice Transformation Program to increase HPV vaccination among PLWH. Guided by the RE-AIM framework, the CHAMPS study will provide training and motivation to HIV providers and clinic staff to recommend and administer the HPV vaccination within three HIV clinics in Georgia. We plan to enroll 365 HIV participants to receive HPV education, resources, and reminders for HPV vaccination. Sociodemographic, HPV knowledge, and vaccine hesitancy will be assessed as mediators and moderators for HPV vaccination. The primary outcome will be measured as an increase in uptake rate in initiation of the HPV vaccine and vaccine completion (secondary outcome) compared to historical baseline vaccination rate (control).
Discussion
The proposed study is a novel approach to address a serious and preventable public health problem by using an efficacious, evidence-based intervention on a new target population. The findings are anticipated to have a significant impact in the field of improving cancer outcomes in a high-risk and aging HIV population.
Trial registration
NCT05065840; October 4, 2021.
Background
HPV-related anal cancer occurs in excess rates among people living with HIV (PLWH) [1], and has been increasing in incidence [1]. Notably, the incidence of anal cancer among men who have sex with men (MSM) is 20- to 40- fold greater relative to non-MSMs [2]. The Human Papillomavirus (HPV) is responsible for 90% of anal cancers where oncogenic HPV type 16 is responsible for 90% of anal cancers [3]. It is presumed the increased risk for anal cancer among PLWH is due to an impaired ability to clear HPV infections and increased reactivation of latent HPV infection. Of note, highly active antiretroviral therapy (HAART) has modest to no effect on HPV clearance or persistence; thus, other mechanisms may be involved that result in cellular immune dysfunction [4].
The safety and efficacy of the HPV vaccine has been evaluated in PLWH and is shown to be safe and highly immunogenic against oncogenic HPV types 16 and 18 [5,6,7,8]. The HPV vaccine also has been shown to decrease the risk of HPV-related anal intraepithelial neoplasia in a sample of MSMs [9]. Thus, anal cancer can be potentially a preventable disease through the use of the HPV vaccine [3]. However, very limited research has been conducted on the uptake of HPV vaccination among PLWH. One study found among a sample of young MSM’s who self-reported as HIV-positive, HPV vaccine initiation was 13.4% [10]. Although uptake is low, studies of the acceptability of the HPV vaccine has been found to be high among high risk groups like MSMs [11,12,13].
The United States’ Advisory Committee on Immunization Practices (ACIP) recommends vaccination up to age 26 years and recently FDA (Food and Drug Association) approved up to age 45 years for women and men [14]. ACIP also advises individuals who are immunocompromised to receive the 3-dose series of the HPV vaccine up to age 26 years of age and with shared clinical decision making for those 26 years and older. The Center for Disease Control and Prevention (CDC) urges catchup vaccination for adults who have not been previously vaccinated and remain vulnerable to develop preventable HPV-related cancers [15]. Yet, there is a dearth of studies that have tailored and implemented evidence-based approaches to promote HPV vaccination among PLWH and eligible for catchup vaccination. Since intervention development is costly, complex, and time consuming, we seek to refine and tailor an existing, evidence-based intervention and integrate in a new population and new setting. The CDC’s 4 Pillars™ Practice Transformation Program (4 Pillars™ Program) is a robust and empirically supported strategic approach that promotes the uptake of adult vaccinations and addresses facilitators and barriers at the patient, provider, and clinic level [16]. The 4 Pillars™ Program incorporates these recommendations via “a menu” of strategies to promote the establishment and maintenance of vaccination into routine practice (Table 1).
The 4 Pillars™ Program has shown to improve vaccination rates among high risk adults in primary care practices that successfully implemented strategies across the program [17, 18]. A randomized controlled cluster trial (RCCT) found the 4 Pillars Program significantly increased HPV vaccination among a cohort of 10,861 adolescent patients in primary care practices [19]. The intervention sites increased baseline HPV vaccination by 10.2 percentage points (PP) versus 7.3 PP in the control sites (p < .001) [19]. Furthermore, another large RCCT of adolescents found the 4 Pillars™ Program significantly increased baseline initiation of HPV vaccination by 17.1 PP (p < .001) and increased HPV completion by 14.8 PP (p < .001) [20]. These findings highlight the effectiveness of the 4 Pillars™ Program to increase HPV vaccination in the general population.
The Advancing HPV vaccination for HIV Positive Adults (CHAMPS) study seeks to expand the success of the 4 Pillars™ Program and tailor, refine, and implement in the HIV positive population, who are at high risk for HPV-related cancers and can obtain the most benefit from the vaccine. The strategies selected from the 4 Pillars™ Program are based on an extensive review of the HIV and related literature (Table 2) [21,22,23,24,25,26,27,28]. The intervention will be implemented in three HIV community clinics in Georgia, USA and enroll n = 365 PLWH, age 18–45 years, from those clinics. Guided by the RE-AIM framework, the proposed specific aims are:
-
1.
Tailor and refine the 4 Pillars™ program for implementation in three HIV community clinics in Georgia.
-
2.
Test the effectiveness of the 4 Pillars™ program as measured by an increase in uptake rate in initiation of the HPV vaccine (primary outcome) and vaccine completion (secondary outcome) compared to historical baseline vaccination rate (control) among PLWH. It is hypothesized after implementation of the 4 Pillars™ program, we estimate an uptake rate of > = 13.5% in initiation of HPV vaccination.
-
3.
Identify mediators and potential moderators (HPV knowledge and awareness and vaccine hesitancy) of the intervention effects on HPV vaccination.
-
4.
Assess the sustainability of the intervention in vaccine uptake post-intervention and assess scalability of the program for wider implementation via a future national randomized control trial.
Methods/design
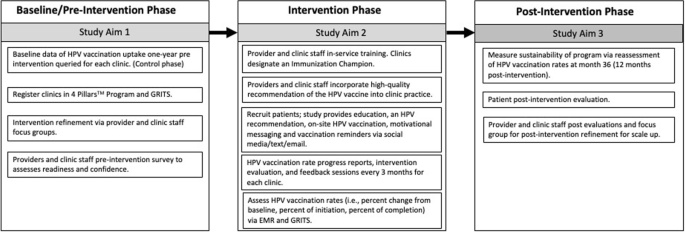
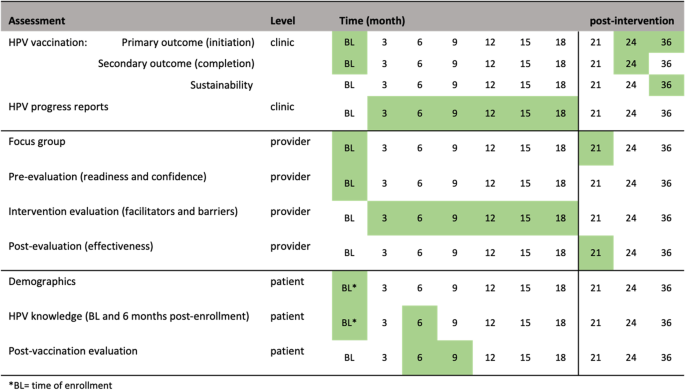
A pre-post intervention study design is used where HPV vaccination initiation and completion rates are measured before and after the intervention across the same clinics and enrolled participants (Fig. 1). HPV vaccination uptake 18 months before intervention will be queried from electronic medical records (EMR) and Georgia Registry of Immunization Transactions and Services (GRITS), which will serve as the historical control. GRITS is a population-based web application containing consolidated demographic and immunization history information. The use of a concurrent control is to reassess the background HPV uptake rate among PLWH during an adjacent time-period to post-intervention and similar population resources. The comparison of HPV vaccination rates pre- and post-intervention in the three selected representative HIV clinics in Georgia will be an exploratory goal of the trial due to the retrospective approach in the control phase and the prospective approach in the post intervention phase. A “within” analysis will be conducted to compare sites both pre- and post-intervention.
Clinic selection and patient eligibility
Three HIV community clinics for this study were selected due to agreement to participate in the study, granted study access to electronic medical records, and willingness to make office changes to increase vaccination rates. Patients will be recruited from these three clinics and enrolled in the study based on the following eligibility criteria: 1) HIV positive; 2) 18–45 years of age; 3) understand English; 4) capable of informed consent; 5) have not received or completed the three dose HPV vaccine; 6) no contraindications to receiving the HPV vaccine (i.e., history of an anaphylactic allergy to latex, an immediate hypersensitivity to yeast, current moderate or severe acute illness, and/or are currently pregnant).
Pre-implementation approach
During the pre-intervention phase of the trial, each clinic site enrolls patients who receive immunizations using the GRITS system. The GRITS system offers a variety of functions for health care providers including recording immunizations, validating immunization history, providing immunization recommendations, producing recall and reminder notices, generating vaccine usage and client reports, and performing data extraction. Clinics will register with the 4 Pillars™ Program and clinic staff will complete a pre-intervention survey that assesses readiness and confidence in increasing HPV vaccination and current vaccination practices. Providers and clinic staff will be asked to participate in a focus group for feedback on tailoring the intervention for their clinic population prior to program implementation.
Clinic-level intervention approach
The 4 Pillars™ Program offers providers and clinic staff evidence-based strategies to increase HPV vaccination uptake via training and educational resources. This program will be refined to provide tailored training and motivation to HIV providers and clinic staff to recommend and administer the HPV vaccine to HIV patients at the infectious disease clinic. Providers and clinic staff who are interested in participating will “enroll” online and complete an electronic informed consent before participating in the focus groups and completing the evaluation surveys. Providers and clinic staff are offered an opportunity to attend an in-service training that will provide education, training, and resources to help increase HPV vaccination at their clinic. Participation in the in-service will be offered to the entire clinic with opportunity for continuing education (CE) units to be earned. The in-service component is delivered under the purpose of quality improvement and does not require informed consent to attend.
Components of the in-service training consist of education and resources related to the 4 Pillars™ program, epidemiology of HPV-related cancers among HIV positive individuals, ACIP’s guidelines for HPV vaccination for immunosuppressed patients, safety profile of the vaccine, and the importance and effectiveness of delivering evidence-based recommendations for HPV vaccination. Providers and clinic staff who are within scope of practice to administer the HPV vaccine are asked to recommend and administer the HPV vaccine to eligible patients during each routine clinic visit. Consenting providers and clinic staff are asked to complete pre-intervention evaluations, an intervention evaluation every 3 months, and post-intervention evaluations via a secured link to complete online. Alternatively, paper copies will be provided to the providers and clinic staff and administered by an Immunization Champion to those who choose not to access the evaluations by email.
Each clinic site identifies an Immunization Champion (a medical assistant, nurse, or clinic manager) who will work and motivate the clinic staff and participate in biweekly updates of progress with the research coordinator. The Immunization Champion (IC) will encourage, remind, and ensure timely documentation of the HPV vaccination within the clinic’s electronic medical records and within GRITS. The IC helps maintain stock and storage of the vaccine and identify and address any issues with the vaccine inventory. The IC assists patients to complete Merck’s Patient Assistance Program to cover vaccination for those who are uninsured and qualify for the program. Lastly, the IC will contact patients on the monthly call list to schedule appointments or assist in reminders to patients to schedule the next visit for the follow up HPV vaccine.
Clinics receive a progress report that documents the clinic’s vaccination progress every three months. The research coordinator schedules group feedback sessions via in-person or webinar with the IC every three months to discuss: 1) the intervention evaluations completed by the providers and staff; 2) to learn of any barriers to HPV vaccination at the clinic; 3) brainstorm strategies to overcome the barriers; and 4) quality assurance of intervention fidelity.
Patient-level intervention approach
Eligible and consenting participants (n = 365) will be part of the intervention group and will receive recommendation for the HPV vaccine from providers and clinic staff. Enrolled participants will also complete a self-administered questionnaire at enrollment on a HIPPA compliant online data management database on a tablet device. The survey questions will include sociodemographic characteristics, knowledge of and attitudes towards HPV, HPV vaccination, and anal cancer, and vaccine hesitancy. Participants will be requested to provide consent for their HPV vaccination history to be verified with electronic EMR and GRITS. Participants will then watch a short video on HPV and HPV vaccination that can be viewed on their phones (or the study’s tablet device) while waiting to be seen. Potential participants will be asked to follow the study’s private Facebook page which offers additional educational information tailored towards individuals with HIV on HPV-related disease and general health promotion, and risk reduction tips. The Facebook page will also utilize Facebook Messenger (commonly known as Messenger). The proposed study will utilize Messenger to send reminders for follow up appointments for the next shot in the series and motivational messaging to encourage and promote receipt of the HPV vaccine. Participants will be contacted to complete a post-evaluation survey administered via online, telephone, or a mailed paper copy at 6–9 months after baseline. Participants will receive $25 incentive for completion of baseline and follow up study activities.
Data analysis plan
Study outcomes
Initiation of the HPV vaccine is the primary outcome endpoint (Fig. 2). Initiation of the HPV vaccine is defined as receiving the first or second immunization from the series. This variable will be measured by electronic medical records and GRITS at baseline (historical control) and 24 months post baseline. We hypothesize the initiation rate will be higher than the historical control rate. Completion of the HPV vaccine is the secondary outcome variable. Completion is defined as receiving all three immunizations from the series, regardless of time. This variable will be measured by electronic medical records and GRITS baseline (control) and 24 months post baseline.
Process evaluation
The RE-AIM (Reach, Effectiveness/Efficacy, Adoption, Implementation, Maintenance) framework will guide planning, implementation, and evaluation of the 4 Pillars™ program. The study’s reach will be estimated from a quantitative perspective by estimating the target population that was exposed to the intervention. The clinic’s patient census data during the period of the implementation phase will be used to estimate the likely reach of the program across sites. Intervention effectiveness and efficacy will be measured by the change in uptake rate of vaccination (i.e., intervention effectiveness). We will calculate the percent change in initiation of the vaccine and percent change in completion of the vaccine from the control phase and 24 months post intervention, after adjusting for demographics differences (age, gender, race, healthcare insurance) in population. Providers and clinic staff will be asked to complete an evaluation of HPV vaccination progress every 3-months. We will also collect qualitative data from feedback sessions with the immunization champions to assess opportunities for and barriers to adoption. The post-evaluation interviews with providers and staff who implemented the program will assess extent of involvement, acceptance of the intervention, implementation fidelity, and extent of organizational spread of the intervention. We will assess the frequency, duration, and the extent to which the intervention was implemented as planned, participation attendance, and costs of implementation, as measured via the intervention and post-intervention evaluations. Intervention sustainability will be measured as the gains or maintenance of HPV vaccination rates post-delivery of the intervention. HPV vaccination rates will be calculated via EMR and GRITS at month 36 (12 months post intervention) and compare to HPV vaccination rates at month 24. Follow up assessments will measure penetration or the extent to which recommendation and administration of the HPV vaccination is integrated within the clinic.
Data analyses of primary outcomes
For analysis of the primary (HPV vaccination initiation) and secondary endpoints (HPV vaccination completion), the uptake rate of HPV vaccination pre- and post-intervention will be estimated separately with a 95% exact confidence interval using all eligible cases from both phases. The one-sample binomial exact test will be used to test whether the rate after the intervention is higher than the baseline rate (P0 = 13.5%). We will also perform the Chi-square test to compare the rate change between control and interventional phases, which will be exploratory. Logistic regression will be used to further adjust background difference in study population in pre- and post- intervention phases. For the longitudinal data collected from the intervention phase, the data structure holds multi-level information from patients, providers, and clinic levels. The goal of the analyses is to identify the factors that might impact HPV uptake from each level of information, which might lead to the future improvement of implementation strategy. Data will be described using summary statistics (e.g., quartiles, median, mean, standard deviation) for continuous variables and marginal distribution (frequency and percentage) for categorical variables. The univariate association with the HPV vaccination (yes vs. no) will be tested in logistic regression for each variable separately. The change of survey response between baseline and a follow-up time point will be tested by paired tests (e.g., paired t-test, McNemar test). Along with data visualization, all above mentioned descriptive and univariate association analyses will be repeated within each of the three clinics. Data will be pooled to build the multilevel analysis models, we mainly consider using the mixed-effect model and/or Bayesian multilevel modeling, in which the random effect will be considered at provider and clinical levels. We will follow the key modeling considerations listed by J.J. Hox [29] to identify the significant mediators and moderators at different levels that impact the uptake of HPV vaccination.
Secondary aims
To assess sustainability, HPV vaccination rates will be calculated at month 36 (12 months post-intervention). The change in HPV vaccination rates between month 24 and month 36 (12 months post-intervention) will be tested by McNemar test. The intervention will be deemed as sustaining its effect if rates of HPV vaccine initiation rates at month 36 remains or increases from month 24’s vaccination rates. To inform future scalability of the program, data from the evaluation and post-evaluation surveys will be described using summary statistics (e.g., quartiles, median, mean, standard deviation) for continuous variables and marginal distribution (frequency and percentage) for categorical variables. The similar analyses will be repeated within each of the three clinics. Additionally, we will conduct a post-intervention focus group consisting of the providers and clinic staff that participated in the program. The qualitative evaluation explore how implementation took place; the barriers to and facilitators of implementation success; ways to address any problems that may have occurred; and recommendations to refine the intervention for scale-up. The focus session will be recorded and transcribed where themes will be extracted and used for adaptation, scale-up considerations, and future research directions.
Statistical power
Based on our preliminary data, we found that HPV vaccination rate is around 13.5% (P0) in general, and another larger study in the literature found a very similar rate of 13.6% [10]. We powered the study to detect an uptake rate > 13.5% after the intervention. Thus, a sample size of 317 achieves 80% power to detect a superiority difference of 5% (PB-P0) using an exact one-sided test with a significance level (alpha) of 0.05. We anticipate a 5% superiority difference is reasonable to achieve with an uptake rate of 18.5% (PB) after the intervention. We will be able to reject the Null hypothesis and claim the uptake rate of > 13.5% after 54 patients have initiated the HPV test. After taking about 15% of the drop-off rate into account, we plan to include 365 participants among 3 clinics for the intervention phase. The calculation was by PASS 2020 for testing superiority of one proportion using the Exact test. The Null hypothesis is P < = 13.5% and the alternative hypothesis is P > 13.5%. For the control phase, we will query all eligible subjects from EMR database among the 3 clinics at 18 months pre-intervention, which could be approximately 2300 subjects [30]. Assuming we end up with the same number of subjects in both control (N = 317) and intervention phase (N = 317), we will have 81% statistical power to detect an HPV uptake rate difference of 9% (22.5% vs. 13.5%) by two-sided Fishers’ Exact Test and under significance level of 0.05. We anticipate such a difference would be feasible based on our best knowledge and the literature. Regarding the patient- level component of the intervention, all incoming eligible patients will be influenced, and hence 365 participants are the minimum number to capture the follow-up information, the actual number of sample size used for the calculation of HPV vaccination rate will be larger as the vaccination status will be captured automatically in EMR without a consent.
Discussion
The underutilization of HPV vaccination is a national problem that has been identified by the President’s Cancer Panel as a serious but correctable threat to the progress against cancer [31]. However, few studies have focused on the high-risk HIV population—an aging population that is increasingly managing other co-morbidities with their HIV diagnoses, including cancer. HPV vaccination is a form of primary cancer prevention that is imperative for a successful cancer control plan that may reduce the untimely death and clinical burden of HIV patients from several potentially vaccine-preventable HPV-related cancers including anal, cervical, vulvar, vaginal, penile, and oropharyngeal cancers. With an aging HIV population, it is an essential public health goal to provide the necessary resources and cancer prevention strategies for PLWH to achieve a normal life expectancy and quality of life. The CHAMPS study is the next step to achieving this goal for high-risk HIV-positive populations.
Trial registration
NCT05065840; Registered on October 4, 2021.
Availability of data and materials
The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
- HPV:
-
Human Papillomavirus
- HIV/AIDS:
-
Human Immunodeficiency virus/ Acquired immunodeficiency syndrome
- PLWH:
-
People living with HIV
- MSM:
-
Men who have sex with men
- HAART:
-
Highly active antiretroviral therapy
- FDA:
-
Food and Drug Administration
- CDC:
-
Centers for disease control and prevention
- PP:
-
Percentage points
- CHAMPS:
-
Advancing HPV vaccination in HIV positive adults
- RE-AIM:
-
Reach, Effectiveness/Efficacy, Adoption, Implementation, Maintenance
- EMR:
-
Electronic medical records
- GRITS:
-
Georgia Registry of Immunization Transactions and Services
- CE:
-
Continuing education
- IC:
-
Immunization champion
- PASS:
-
Power analysis & Sample size
References
Khandwala P, Singhal S, Desai D, Parsi M, Potdar R. HIV-Associated Anal Cancer Cureus. 2021;13(5):e14834.
Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54(7):1026–34.
De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta analysis. Int J Cancer. 2009;124(7):1626–36.
Shrestha S, Sudenga SL, Smith JS, Bachmann LH, Wilson CM, Kempf MC. The impact of highly active antiretroviral therapy on prevalence and incidence of cervical human papillomavirus infections in HIV-positive adolescents. BMC Infect Dis. 2010;10(1):295.
Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–11.
Palefsky JM, Gillison ML, Strickler HD. HPV vaccines in immunocompromised women and men. Vaccine. 2006;24:S140–S6.
Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. 2013;57(5):735–44.
Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53.
Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85.
Meites E, Markowitz LE, Paz-Bailey G, Oster AM, Group NS. HPV vaccine coverage among men who have sex with men–national HIV behavioral surveillance system, United States, 2011. Vaccine. 2014;32(48):6356–9.
Cummings T, Kasting ML, Rosenberger JG, Rosenthal SL, Zimet GD, Stupiansky NW. Catching up or missing out? Human papillomavirus vaccine acceptability among 18- to 26-year-old men who have sex with men in a US National Sample. Sex Transm Dis. 2015;42(11):601–6.
Nadarzynski T, Smith H, Richardson D, Pollard A, Llewellyn C. Perceptions of HPV and attitudes towards HPV vaccination amongst men who have sex with men: a qualitative analysis. Br J Health Psychol. 2017;22(2):345–61.
Reiter PL, Brewer NT, McRee A-L, Gilbert P, Smith JS. Acceptability of HPV vaccine among a national sample of gay and bisexual men. Sex Transm Dis. 2010;37(3):197.
FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. 2018.
Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2014;63(5):1–30.
Abdool Karim SS, Passmore JS, Baxter C. The microbiome and HIV prevention strategies in women. Curr Opin HIV AIDS. 2018;13(1):81–7.
Nowalk MP, Moehling KK, Zhang S, Raviotta JM, Zimmerman RK, Lin CJ. Using the 4 pillars™ to increase vaccination among high-risk adults: who benefits? Am J Manag Care. 2017;23(11):651.
Zimmerman RK, Brown AE, Pavlik VN, Moehling KK, Raviotta JM, Lin CJ, et al. Using the 4 pillars practice transformation program to increase pneumococcal immunizations for older adults: a cluster-randomized trial. J Am Geriatr Soc. 2017;65(1):114–22.
Zimmerman RK, Moehling KK, Lin CJ, Zhang S, Raviotta JM, Reis EC, et al. Improving adolescent HPV vaccination in a randomized controlled cluster trial using the 4 pillars™ practice transformation program. Vaccine. 2017;35(1):109–17.
Zimmerman RK, Raviotta JM, Nowalk MP, Moehling KK, Reis EC, Humiston SG, et al. Using the 4 pillars™ practice transformation program to increase adolescent human papillomavirus, meningococcal, tetanus-diphtheria-pertussis and influenza vaccination. Vaccine. 2017;35(45):6180–6.
Apaydin KZ, Fontenot HB, Shtasel D, Dale SK, Borba CP, Lathan CS, et al. Facilitators of and barriers to HPV vaccination among sexual and gender minority patients at a Boston community health center. Vaccine. 2018;36(26):3868–75.
Gorbach PM, Cook R, Gratzer B, Collins T, Parrish A, Moore J, et al. Human papillomavirus vaccination among young men who have sex with men and transgender women in 2 US cities, 2012–2014. Sex Transm Dis. 2017;44(7):436.
Klosky JL, Hudson MM, Chen Y, Connelly JA, Wasilewski-Masker K, Sun C-L, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017;35(31):3582.
Reiter PL, Katz ML, Bauermeister JA, Shoben AB, Paskett ED, McRee A-L. Recruiting young gay and bisexual men for a human papillomavirus vaccination intervention through social media: the effects of advertisement content. JMIR Public Health Surveill. 2017;3(2):e33.
Subasinghe AK, Nguyen M, Wark JD, Tabrizi SN, Garland SM. Targeted Facebook advertising is a novel and effective method of recruiting participants into a human papillomavirus vaccine effectiveness study. JMIR Res Protocols. 2016;5(3):e154.
Wells JS, Flowers L, Paul S, Nguyen ML, Sharma A, Holstad M. Knowledge of anal cancer, anal cancer screening, and hpv in hiv-positive and high-risk hiv-negative women. J Cancer Educ. 2020;35(3):606–15.
York JM, Klosky JL, Chen Y, Connelly JA, Wasilewski-Masker K, Giuliano AR, et al. Patient-level factors associated with lack of health care provider recommendation for the human papillomavirus vaccine among young Cancer survivors. J Clin Oncol. 2020;38(25):2892–901.
Rand CM, Vincelli P, Goldstein NP, Blumkin A, Szilagyi PG. Effects of phone and text message reminders on completion of the human papillomavirus vaccine series. J Adolesc Health. 2017;60(1):113–9.
Hox JJ, Moerbeek M. Van de Schoot R. multilevel analysis: techniques and applications: Routledge; 2017.
Georgia Department of Health. HIV surveillance summary, Georgia 2018. In: Section HAE, editor. 2020.
Panel PsC. HPV vaccination for cancer prevention: progress, opportunities, and a renewed call to action. A report to the president of the United States from the chair of the President’s Cancer Panel 2018.
Acknowledgments
The 4 Pillars™ Immunization Toolkit Materials are an evidence-based educational and support program developed by the University of Pittsburgh and funded by the Centers for Disease Control to increase immunizations.
Funding
This study was supported by National Institutes of Health, National Institute of Nursing Research, R01NR020154. The funders had no role in the study design, data collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
JW is responsible for the study design, oversight of the study and draft of the manuscript. JW, JK, YL, TG contributed to the writing and critical review of the manuscript. YL contributed the statistical analysis plan of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval from Emory University’s Institutional Review Board and ethical approval from the participating clinic sites was obtained before data collection (IRB00002469). All eligible participants will provide written informed consent before study enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wells, J., Klosky, J.L., Liu, Y. et al. An overview of implementing an evidence based program to increase HPV vaccination in HIV community clinics. BMC Public Health 22, 1696 (2022). https://doi.org/10.1186/s12889-022-14100-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14100-0